 By Robin Raiford and Anantachai (Tony) Panjamapirom, iHealthbeat.org
By Robin Raiford and Anantachai (Tony) Panjamapirom, iHealthbeat.org
To meet many of the meaningful use requirements, providers must capture, store and share clinical data mostly in a specified electronic, structured and coded format. Having undergone a major ramp-up data capture in Stage 1, providers will continue to experience the increased pressure and intensity in both quantity and quality of required data elements. Providers should view this mandate, as an opportunity to transform their data collection process and develop plans to sustain providers’ agility needed to successfully demonstrate meaningful use as the future stages will only bring additional data elements and more complex requirements.
Devil in the Details — More Data To Capture
In Stage 1, providers were able to meet key objectives of meaningful use with a single piece of data. For example, a single entry in a problem, medication or medication allergy list met the measure threshold.
Starting in 2014, this is no longer the case. All participants, regardless of their stage of meaningful use, need to ensure the accuracy and completeness of the data as they must start sharing more of these data with patients and other providers. There are three objectives that require significant data capture:
- View, Download, and Transmit (VDT);
- Transition of Care (TOC); and
- Clinical Summaries (eligible professionals only).
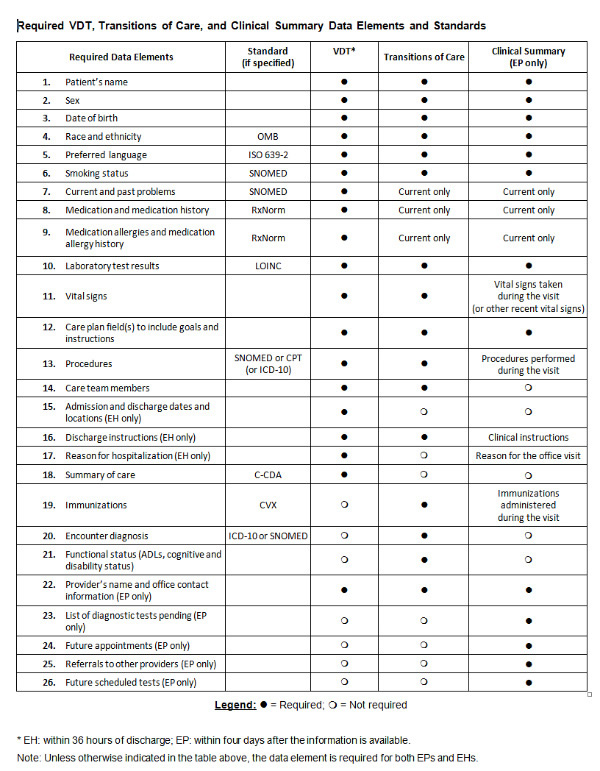
Specifically, providers must make available all current and past problems, medications and medication allergies to meet the requirement of the VDT objective. In addition, new types of data elements — such as care plan fields, including goals and instructions — will be required for all three objectives.
Because of the regulatory changes in the Stage 2 final rule, Stage 1 providers in 2014 and beyond will get a real taste of more complex Stage 2 measures. The most significant change requires Stage 1 eligible professionals (EPs) and eligible hospitals (EHs) to meet Measure 1 of the VDT objective, where providers must make available required data elements for patient electronic access within 36 hours for EHs and four days for EPs. Stage 2 EPs and EHs also must meet Measure 2 of the VDT objective, which requires 5% of unique patients discharged during the reporting period to view, download or transmit their available information. The VDT objective substantially increases the number of required data elements, which must be accessible online in a specified timeframe.
Collectively for EPs and EHs, there are 26 required data elements in the VDT, TOC or Clinical Summaries (EPs only) objectives, which is significantly higher than the five or six minimum required data elements for Electronic Copy of Health Information (for EPs and EHs) and Timely Electronic Access (EPs only) objectives in Stage 1.
Furthermore, not only does the number of data elements increase, CMS also requires some of these data to be coded with specific vocabulary standards, some of which are new. For example, preferred language must be coded in International Organization for Standardization (ISO) 639-2 and medication allergy in RxNorm. In addition, Systematized Nomenclature of Medicine (SNOMED) is a new standard for smoking status.
Major Workflow and Change Management Implications
When the EHR Incentive Programs were first launched, some health care organizations perceived them as an “IT project” because the name reflected the presence of technology. However, organizations quickly realized that this “IT-enabled initiative” focused heavily on workflow process improvement and change management throughout the organization. As signaled in Stage 2, future stages of meaningful use will drastically drive toward capturing real-time, accurate and complete data, and thus clinical staff adoption of workflow and compliance to policy will be increasingly important.
To meet Stage 1 requirements, a number of hospitals rely on health information management staff to retrospectively code required data elements. However, as discussed above, the Stage 2 regulatory changes drive the intensity of data capture from both magnitude and shortened turnaround time perspectives. Henceforth, a reliance on back-end coding will likely be unsustainable and could prevent providers from successfully demonstrating meaningful use. In responding to such regulatory change, the majority of health care organizations are redesigning workflows and making internal policy changes to facilitate real-time, point-of-care data capture. If not already doing so, providers should begin conduct a gap analysis and assess how many of the required data elements are currently captured and coded. They should then subsequently develop a remediation plan for missing data elements with timeline and assigned responsibility.
Click image to enlarge.
For further information regarding all required data elements for VDT, TOC, Clinical Summaries, and various templates for the Consolidated Clinical Data Architecture (C-CDA), see the Office of the National Coordinator for Health IT’s Companion Guide Requirements Mapping Spreadsheet.