EHR Interoperability: The Structured Data Capture Initiative
 Dr. Doug Fridsma / Chief Science Officer and Director, Office of Science & Technology
Dr. Doug Fridsma / Chief Science Officer and Director, Office of Science & TechnologyMeaningful Use (MU) Stage 1 and MU Stage 2 established the initial portfolio of interoperability standards to support exchanging data from one EHR to another EHR.
As more and more people recognize the value of standardized data, people are looking to take advantage of the power EHR’s offer to support: MU, quality measure reporting, and other important activities to aid in patient care.
Electronic, standardized, and sharable data means that there are many other uses that we might consider for the data contained within an EHR. For instance:
- Patient safety advocates may want to use EHR systems to collect patient safety information, leveraging existing standards like the AHRQ “common format” for patient safety reporting
- Providers and researchers may want to use the EHR systems to collect data for clinical research, including patient-centered outcomes research, and to identify patients who could benefit from participating in a research study
- Providers may want to give referrals to their patients for community services, like smoking cessation or weight management programs, after discussing these topics with them during an office visit
- Providers working with disease surveillance case report forms may wish to collect additional information about reportable conditions, such as infectious diseases
- Provider’s office staff can use EHR’s to gain pre-authorization of certain kinds of medical devices where health payers may want to leverage clinical information collected in EHRs to support additional review of expensive medical equipment.
Although health care providers use various sources and methods to capture and synthesize patient-level data, EHRs are a data source with tremendous potential to provide timely and relevant data in a form that is:
- Usable for quality and safety improvement
- Population health
- Research (sometimes labeled “secondary” use) [1]
EHR Interoperability: The Benefits of Structured Data Capture
It would be challenging to include every possible data element (as important as it may be) in the core MU data elements. If we do so, we risk overwhelming providers, vendors, or others with the complexity and scope of the standardized data that EHRs would be required to collect.
A solution to this problem is being explored in the latest new initiative in the S&I Framework – the structured data capture (SDC) initiative.
The goal of the initiative is to identify how EHR interoperability technology can be used to:
- Access a template that contains structured data, which is sometimes called common data elements (CDEs)
- Automatically populate the template with the correct CDEs from existing EHR data
- Store or transmit the completed template to the appropriate organization or researcher
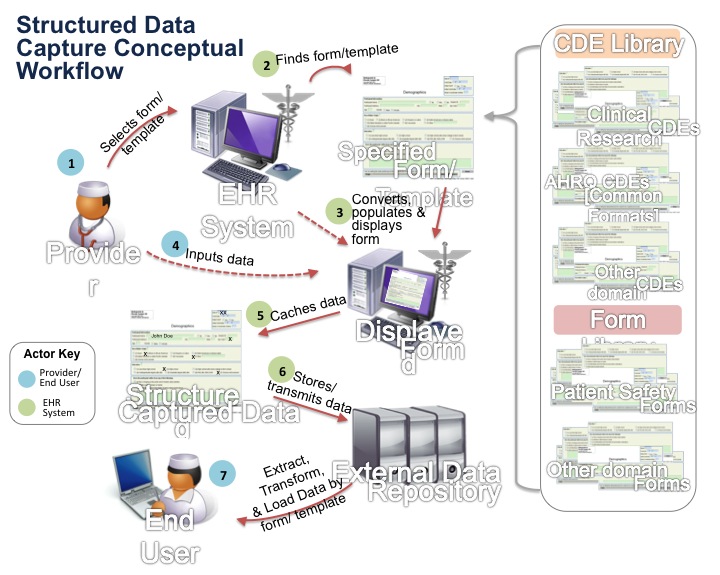
Thankfully, there has been significant work on CDEs already, so like other S&I framework initiatives, we expect that the SDC Initiative will leverage existing EHR interoperability standards, harmonize them, and agree on a common approach to support structured data capture.
A list of the standards that already exist is available on the SDC Initiative wiki page here.
ONC’s Work on EHR Interoperability Standards
ONC is working with the National Library of Medicine and the NIH to:
- Identify an initial core research set of CDEs that could be used by the SDC project
- Pilot how different agencies and organizations can coordinate and govern this core set of CDEs
We are also working with AHRQ to leverage their work on the common format for safety reporting.
Given the significant investments that have been made in EHR adoption and interoperability in the last 4 years, enabling structured data capture within EHRs is poised to be a critical way to integrate EHR data into a variety of health services and clinical research activities.
Future stages of MU will put a “down payment” on learning the health system to support health care quality, research, and overall public and population health. The SDC initiative will support the secure, trusted, patient-centered integration of the EHR into other parts of the learning health care system such as:
- Research consortia
- Registries
- Bio repositories
Success in the SDC project will give us four important interoperability results:
- A standard definition (and structure) for what a data element (CDE) is
- A standard way to collect these data elements into templates
- A standard way to automatically populate the CDEs with data from an EHR
- A standard way to access, display, and store the data.
Ultimately these results will improve access to standardized electronic versions of data collection instruments for use in a variety of research and clinical reporting functions.
Our intent here is not to increase the data collection burden on providers or those entities charged with gathering this type of information.
Our focus is enabling the integration of these instruments into EHRs so that duplicate data entry is reduced throughout the workflow, and most importantly, the data collected is comparable and more useful across multiple groups—from researchers, clinicians, payers, and public health agencies to patients and their caretakers.
I hope that you will consider joining and participating in this exciting initiative!
Dr. Doug Fridsma is the director of the Office of Standards and Interoperability and the Acting Chief Scientist in the Office of the National Coordinator for Health Information Technology. This article post was first published on the ONC Health IT Buzz blog and re-published here with permission.
